Background: Iptacopan is the first oral proximal complement inhibitor that targets factor B to selectively inhibit the alternative pathway (AP) of the complement system. Iptacopan is currently being investigated in Phase III trials in PNH, C3G and IgAN. AP biomarkers can be used to evaluate the inhibitory effect of iptacopan. Wieslab ® ex vivo assay measures the amount of soluble C5b-9 (sC5b-9) formation in serum after AP activation. Plasma sC5b-9 is the final product of complement activation and is also known as the membrane attack complex. Plasma Bb levels increase following AP activation.
Aim: To characterize exposure-response relationships between iptacopan and AP biomarkers Wieslab ®, Bb and sC5b-9 to rationalize the iptacopan dose selection.
Methods: This analysis was based on final data from a Phase I clinical trial enrolling healthy volunteers (X2101 [EUDRACT2015-005567-16]) and 4 Phase II trials enrolling pts with PNH (X2201 [NCT03439839] and X2204 [NCT03896152]), C3G (X2202 [NCT03832114]) and IgAN (X2203 [NCT03373461]). In these trials, oral iptacopan was administered in doses ranging from 5-400 mg single dose and from 10-200 mg twice daily (bid). A longitudinal mixed-effects modeling approach was applied to the AP biomarkers used in this study: serum Wieslab ® assay, plasma Bb and plasma sC5b-9. Direct response sigmoid Emax models were considered as most appropriate and biologically plausible for all biomarkers, reflecting the rapid biomarker response following exposure to iptacopan. The exposure metric driving the response was the observed time-matching pharmacokinetic (PK) data. A population PK (popPK) model built on bid dose levels (10-200 mg) using data from pts (not healthy volunteers) simulated steady state exposure over 6 months of iptacopan bid dosing in each population.
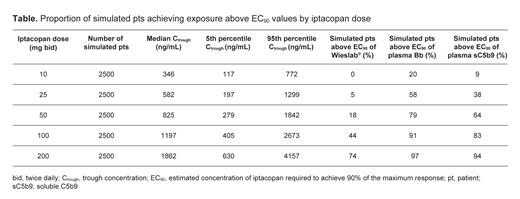
Results: The modeling dataset contained 247 participants, of which 88 (36%) were healthy volunteers, 29 (12%) were pts with PNH, 27 (11%) were pts with C3G, and 103 (42%) were pts with IgAN. Overall, 69 (28%) participants were female, and the median age (range) was 38 (18-78) years. A total of 2317, 2296 and 2279 samples for Wieslab ®, Bb and C5b-9, respectively, were used in the modeling, with the same number of time-matched PK samples. The biomarker responses decreased monotonically with saturation of effect and without apparent time lag, which was expected based on the mechanism of iptacopan. An exposure-dependent decrease in Wieslab ® was seen in pts and healthy volunteers, with estimated iptacopan concentrations required to achieve 90% of the maximum response (EC 90) being 1281 ng/mL (90% confidence interval [CI] 1207, 1418) and 706 ng/mL (90% CI 653, 788), respectively. Based on simulations from the popPK model, this exposure was expected to be reached at steady state trough concentration (C trough) by an estimated 74% of pts at 200 iptacopan mg bid, compared with 44% and 18% receiving 100 mg bid and 50 mg bid, respectively ( Table). The interindividual variability of Wieslab ® response decreased as iptacopan dose increased to 200 mg bid and C trough to ≥1300g/mL. A clear decrease from baseline in plasma Bb was seen following iptacopan treatment, with an EC 90 value of 521 ng/mL (90% CI 268, 912). Based on simulations from the popPK model, this exposure was expected to be reached by 97% of pts treated with iptacopan 200 mg bid, compared with 91% and 79% receiving 100 mg bid and 50 mg bid, respectively ( Table). For sC5b-9, the EC 90 value was 682 ng/mL (90% CI 503, 894) across all participants. This exposure was expected to be achieved by 94%, 83% and 64% of pts receiving iptacopan 200 mg bid, 100 mg bid and 50 mg bid, respectively ( Table). Age, weight, sex and ethnicity did not affect the biomarker responses.
Conclusions: In this analysis, iptacopan 200 mg bid was the dose at which the highest proportion of participants achieved EC 90 values for the AP biomarkers Wieslab ®, Bb and sC5b9. Other than pts having higher EC 90 values than healthy volunteers for Wieslab ®, no differences were found in EC 90 values between the trial populations, demonstrating that no dose adjustment is needed between them. A response plateau was achieved by the iptacopan 200 mg bid dose for Wieslab ® and lower doses for Bb and C5b-9, suggesting higher doses would not lead to greater responses. In summary, these data support the use of oral iptacopan at 200 mg bid to achieve rapid, substantial and sustained inhibition of the complement AP in pts with PNH, C3G or IgAN.
Disclosures
Baltcheva:Novartis Pharma AG: Current Employment. Bartels:Novartis Pharma AG: Current Employment, Current equity holder in publicly-traded company. Valentin:Novartis Pharma AG: Current Employment, Current holder of stock options in a privately-held company. Schmouder:Novartis Pharmaceuticals Corporation: Current Employment, Current holder of stock options in a privately-held company. Junge:Novartis Institutes of Biomedical Research: Current Employment, Current equity holder in publicly-traded company. Yu:Novartis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal